- Healthy Aging, Happy Living! NYCU Launches Training Program on Age-Friendly Housing
- NYCU GIA Professors Present Taiwan’s Innovative and Sustainable Spirit in Japan
- NYCU ECE and IEEE Taipei Section Host 2025 AI Computing Workshop to Explore the Future of High-Performance AI Systems
- NYCU Achieves Global Leadership in Industry Collaboration in THE World University Rankings 2026
- NYCU Asfaloth-2025 Rocket Launched: First Integration of TASA’s MLP Marks a Milestone in Academic Space Research
How Cells “Talk” to Their Surroundings: NYCU Study Uncover Liquid Droplet Mechanism Behind Cilia Formation
(中央社訊息服務20251107 14:58:11)National Yang Ming Chiao Tung University (NYCU) researchers have unveiled how cells build their microscopic “antenna” — a slender, hair-like structure known as the primary cilium, which plays a crucial role in sensing the environment. The study reveals that two key proteins responsible for cilia formation can fuse, much like liquid droplets, through a process called liquid–liquid phase separation (LLPS), offering new insights into cellular communication and potential treatments for ciliopathies. The study, titled “Phase separation of TTBK2 and CEP164 is necessary for ciliogenesis,” was published in Cell Reports.
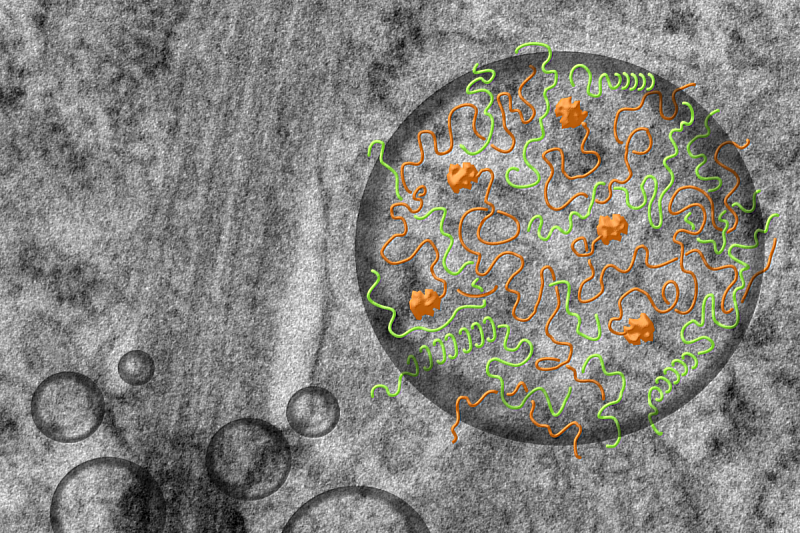
The primary cilium, hundreds of times thinner than a human hair, acts as a cell’s radar. When damaged, cells lose their ability to perceive external signals, leading to developmental disorders such as microcephaly and other genetic diseases. While scientists have long known that specific cellular structures and proteins are required for cilia assembly, the mechanism by which certain proteins interact to trigger the process has remained a mystery — until now.
Led by Professor Won-Jing Wang and Jie-rong Huang from NYCU’s Institute of Biochemistry and Molecular Biology, the research team used human retinal pigment epithelial cells to investigate how two cilia-associated proteins, TTBK2 and CEP164, interact. They discovered that these proteins do not bind like puzzle pieces or “lock and key” models, which are typical of structured proteins. Instead, they join through LLPS — a biochemical phenomenon where proteins with intrinsically disordered regions attract each other via electrostatic forces to form liquid-like condensates.
“Liquid–liquid phase separation (LLPS) has only recently gained widespread attention,” said Prof. Huang. “Scientists used to believe that only structured regions of proteins could interact. We now know that even disordered regions can combine through this liquid droplet behavior, redefining how we understand protein organization in cells.”
Prof. Wang added, “Cilia are fascinating organelles. Some types, like the primary cilium, act as sensory antennas — such as those found on retinal cells — while others, like motile cilia, help move cells or fluids, as seen in sperm tails and respiratory tracts. Our discovery provides the first molecular evidence that protein phase separation drives cilia formation.”
Mutations in the TTBK2 gene are known to cause neurodegenerative conditions such as spinocerebellar ataxia, a form of cerebellar degeneration. The NYCU team’s discovery sheds light on how abnormal phase separation may disrupt cilia assembly, opening a potential pathway toward developing therapeutic strategies for ciliopathies and related diseases.
This groundbreaking finding not only deepens our understanding of how cells construct their sensory machinery but also highlights the intricate beauty of biological self-organization — where even shapeless molecules can come together to build life’s most delicate structures.






